Worldwide Market, First-in-Class
Tested In Phase II (PoC) clinical trial

KT-110 Timeline
General description
- Status: Phase II completed / Phase III designed
- Indication: Alcohol addiction
- Targets: 5-HT2 and alpha1-adrenergic receptors, which are central in the addiction processes. Indeed, they are involved in the activation of dopamine-producing neurons and in their action on the circuit of the reward. Their simultaneous inhibition allows to oppose the addicting effects of toxicomanogenous products by avoiding the side effects such as those produced by the antipsychotics.
Timeline
The goals planned for phase 2 proof of concept study were all reached:
- Confirm KT-110 safety
- Validate the pharmacological concept of recoupling reward system control
- Identify best responder population
- Determine the optimal dose
- Shape further development
Preclinical studies on other indications are pursued

KT-110 is a First in Class
innovative pharmaceutical composition
The antagonists Prazosin and Cyproheptadine entering the composition of KT-110 were strictly selected for their efficiency and on the basis of the following criteria:
- Regarding Prazosin, this is the gold standard among alpha-1 antagonists.
-
Regarding Cyproheptadine, there are 3 therapeutic classes of 5-HT2 antagonists:
- antipsychotics
- antidepressants
- antipsychotics
- first generation antihistamines
In order to avoid confusion and adverse effects, antipsychotics and antidepressants were not considered. And among all first generation antihistamines, Cyprohetadine is the most used.
Synergy and IP :
- KT-110 composition is better than other drugs such as acomposate, naltrexone or nalmefene.
- Patented protection of the composition
- Patent on new BID formulation is being filed
Safety:
- Perfect knowledge of the components adverse effects
Preclinical trials
PRECLINICAL DATA
Selected composition:
Cyproheptadine : Antagonist of 5HT2 and 5HT1C receptors used in the treatment of allergy
Prazosin : Antagonist of alpha1-adrenergic receptors used in the treatment of arterial high blood pressure
Addiction to alcohol:
Effects of the combination on the alcohol intake and on preference to alcohol.
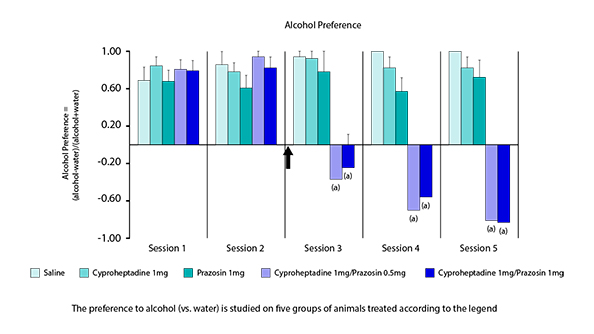
Cyproheptadine + Prazosin in combination (KT-110 of Kinnov Therapeutics) reverses preference to alcohol and induces a strong alcohol aversion. Other studies show better efficacy of KT-110 than available products.
clinical trials
Interview : Opinion of addiction experts on AUD & KT-110, following phase 2 clinical trial results
Five addiction experts (Henri-Jean Aubin, Karl Mann, Wim van den Brink, Antoni Gual and Raymond Anton), provide insights in alcohol use disorder (AUD) and its current treatments, and how new treatment KT-110 would benefit AUD patients.
Henri-Jean Aubin is Professor of Psychiatry and Addictology at Hôpital Pitié-Salpétrière in Paris, France. He was the Principal Investigator in KT-110 phase 2 trial.
Karl Mann is Professor Emeritus at Central Institute of Mental Health, University of Heidelberg, Germany.
Wim van den Brink is Professor Emeritus of Psychiatry and Addiction at Academic Medical Center, University of Amsterdam, The Netherlands.
Antoni Gual is Head of the Addictions Unit Neurosciences Institute at University of Barcelona, Spain.
Raymond Anton is Professor Emeritus of Psychiatry and Addictions at Medical University of South Carolina, USA.
COCKTAIL Phase 2b study of KT-110 on alcohol addiction
Study enrolled 154 subjects in 35 french sites and showed a significant reduction in the high dose group compared to the placebo group, as well a good safety profile.
Phase 2b study on alcohol addiction was conducted for Kinnov Therapeutics by Pr. Alain Puech Selected CRO: ECSOR (www.ecsor.com), highly experienced in coordinating clinical studies in the addiction field. The clinical trial manager was Lucas Biela, who has past experience with study management in CROs, biotechs and pharmaceutical companies.
Principal investigator: Prof. Henri-Jean Aubin, Hôpital Paul Brousse, Villejuif
DSMB Pdt: Dr. Ivan Berlin, Hôpital Pitié-Salpêtrière, Paris
Both are internationally renowned for their competence in alcohol and tobacco addiction studies
list of sites available here